PASSMED公式LINEの登録者特典|当サイトに掲載している図表の元データ&学習支援AI 薬科GPTをプレゼント♪

This page is information for Japan!
On June 26, 2023 , LITFULO Capsules (ritrecitinib) was approved for the treatment of alopecia areata !
Pfizer | News Release
Basic information
| product name | LITFULO Capsules 50mg |
| common name | ritresitinib tosilate |
| Origin of product name | Derived from the meaning of full enjoyment of daily life. |
| manufacturing and sales | Pfizer Japan Inc. |
| Efficacy/effect | Alopecia areata (limited to intractable cases where hair loss is widespread) |
| Dosage and dosage | The usual dosage for adults and children aged 12 years or older is 50 mg of ritrecitinib orally administered once daily. |
Olumient (baricitinib) has already been approved as an oral JAK inhibitor for alopecia areata.
LITFULO is a drug that has the characteristic of inhibiting not only JAK3 but also TEC family kinases !
I think it can be expected as a new treatment option.
This time, along with alopecia areata, I will explain the mechanism of action and evidence of LITFULO (ritrecitinib) ♪
alopecia areata
Alopecia areata is an acquired disease caused by stress.
The basic type is a single type in which hair is removed in a circle like a coin, but there are also multiple types that occur in several places, hair loss over the entire head, and hair loss over the whole body.
The main categories are as follows.
- Normal type (single type, multiple type)
- Whole head type: Hair loss from the entire head
- Generalized type: Whole body hair loss
- Serpentine type: The hairline comes out in a strip
Normally, it is said that 50 to 100 hairs fall out a day, but the same amount is growing and growing. This process of hair growth, growth, and shedding is called the “ hair cycle” .
The key to alopecia areata is the transition from the telogen phase to the anagen phase.
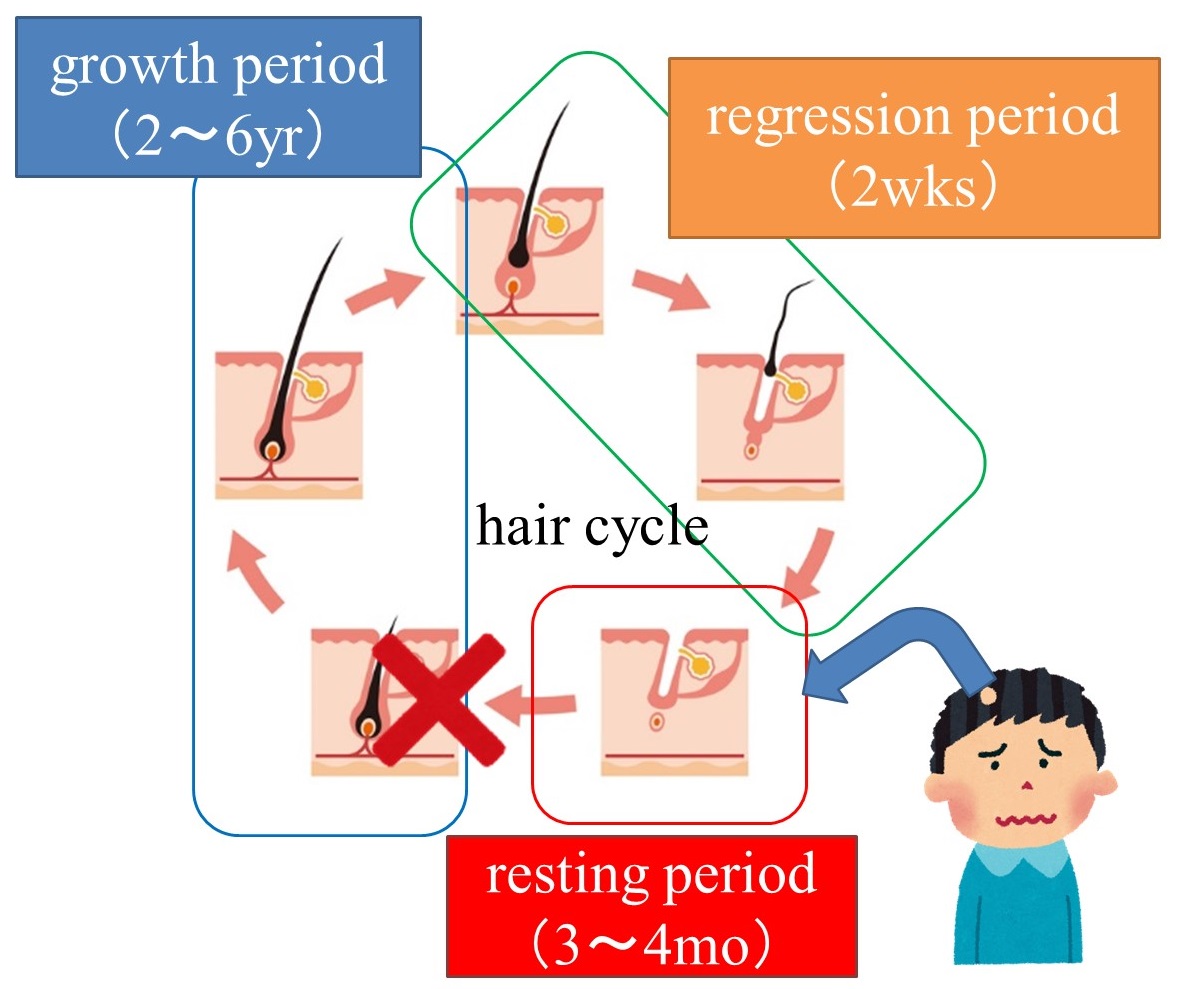
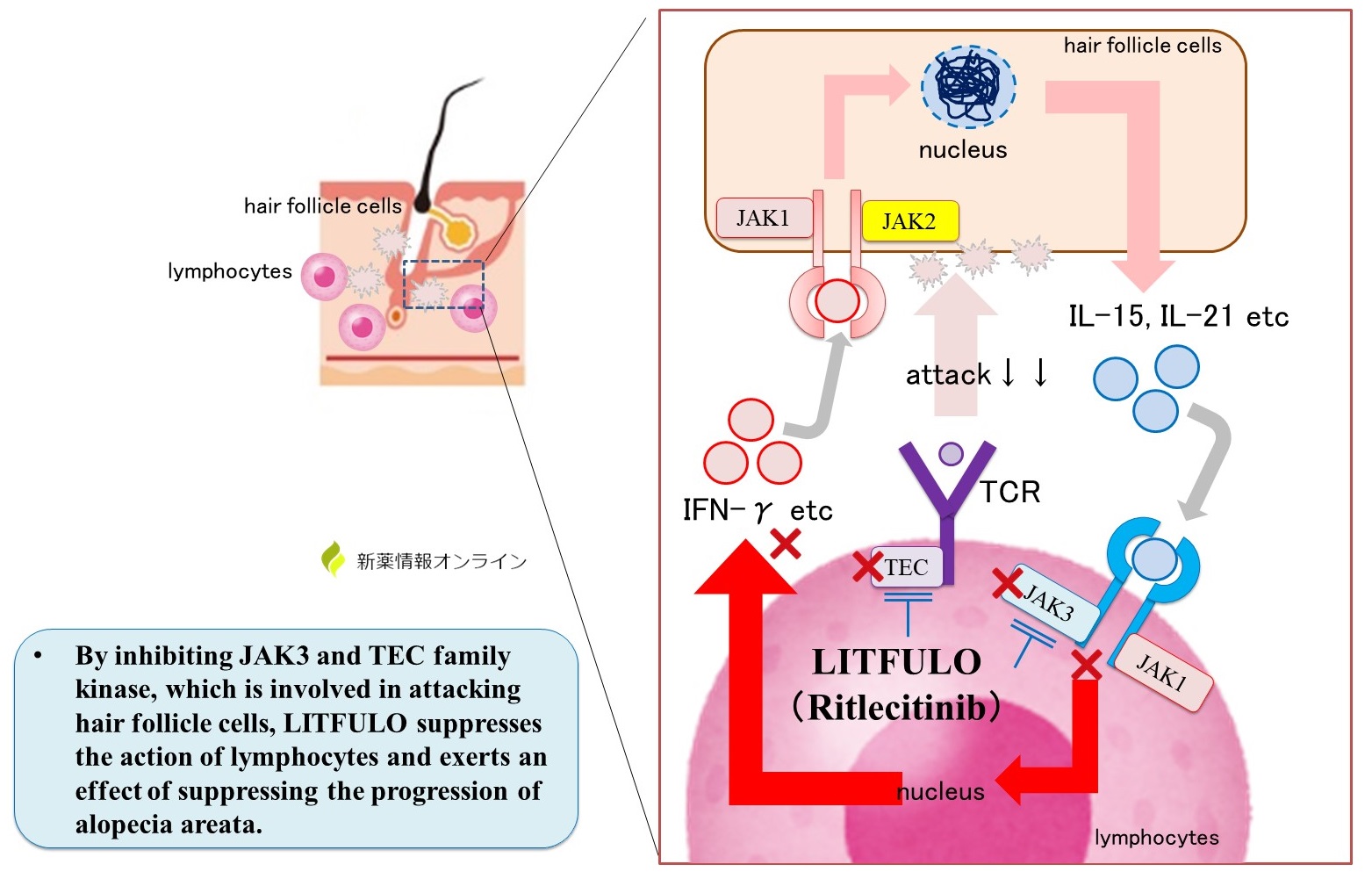
Stress causes lymphocytes to attack hair follicles . As a result, hair follicles are thought to shrink and fail to enter the anagen phase, resulting in hair loss.
The inflammatory cytokines IL-15 and IL-21 are known to be involved in lymphocyte attack, although the exact mechanism is unknown.
IL-15 and IL-21 are produced by hair follicle cells, and IL acts on lymphocyte receptors to promote IFN-γ production and activate hair follicle attack. By acting on hair follicle cells, IFN-γ further promotes the production of IL-15 and IL-21.
JAK (Janus kinase) is a tyrosine kinase present in receptors on which IL-15, IL-21, and IFN-γ act.
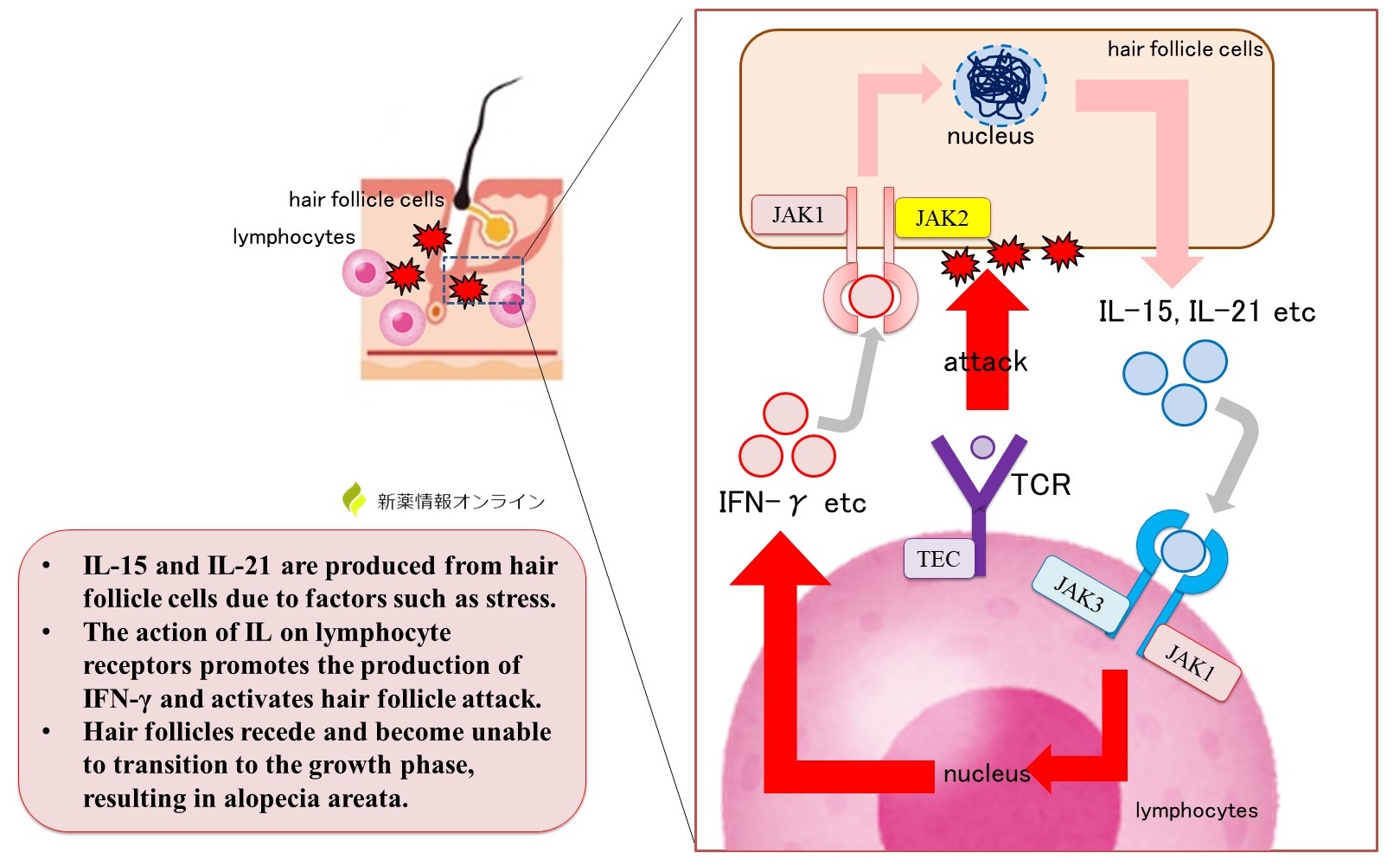
In addition, TCR (T-cell receptor) on the membrane surface of lymphocytes is thought to be involved when lymphocytes attack hair follicle cells. TEC family kinases are present in the TCR.

treatment
Alopecia areata is divided into the "acute stage (progressive stage)" in which the lesion rapidly expands after becoming aware of the symptoms of hair loss, and the "fixed stage" in which spontaneous regeneration is not observed even after six months of hair loss symptoms. are treated differently. 2-3)
It is also important whether the area of hair loss is 25% or less.

| Area of depilation | Fixed phase treatment (recommendation) |
| less than 25% |
|
| 25% or more |
|
Local steroid injection therapy is a local injection of a mixture of Kenacort A suspension (triamcinolone acetonide) and a local anesthetic. However, as a general rule, it is not recommended for children .
In local immunotherapy, chemical substances (reagents) called SADBE (squaric acid dibutylester: 3,4-dibutoxy-3-cyclobutene-1,2-dione) and DPCP (diphenylcyclopropenone) are externally applied to the hair loss site, It is a treatment that improves alopecia areata by changing the immune balance by repeatedly causing contact dermatitis (rash). Both SADBE and DPCP are not covered by insurance because they are not medicines .
In addition, topical steroids, glycyrrhizin (glycyrrhizin/glycine/methionine combination tablets), oral administration of antihistamines, etc. are recommended with a recommendation level of C12), but the actual situation is that there is no clear evidence .
Meanwhile, in 2022, the JAK inhibitor Olumient was expanded for the treatment of alopecia areata, and it became a hot topic.
Mechanism of Action of LITFULO Capsules (ritrecitinib)
LITFULO is a drug that inhibits JAK3, a receptor on which IL-15 and IL-21, which are involved in alopecia areata, act . Inhibition of JAK3 in lymphocytes suppresses lymphocyte activation and suppresses IFN-γ production.
In addition, by simultaneously inhibiting the TEC family kinase of TCR, which is involved when lymphocytes attack hair follicle cells, it suppresses the action of lymphocytes, improving symptoms and suppressing the progression of alopecia areata.

Evidence introduction: ALLEGRO-IIb/III study
We introduce the clinical study (ALLEGRO-IIb/III study) that served as the evidence.
This study is an international Phase IIb/III clinical study comparing LITFULO with placebo in patients aged 12 years and older diagnosed with severe alopecia areata with a Severity of Alopecia Tool (SALT) score of 50 or higher. (including Japanese).
The primary endpoint was the ratio of "SALT score 20 or less at 24 weeks", and the results are as follows (excerpt).
| Placebo group | LITFULO 50mg group |
|
| Proportion of SALT scores ≤20 at 24 weeks | 2% | twenty three% |
| Difference from placebo: p<0.0001 | ||
All treatment groups other than the LITFULO 10mg group showed significant improvement compared to the placebo group!
Although the efficacy at 24 weeks was higher with LITFULO 200mg, the long-term results after 40 weeks were not significantly different from those of the 50mg group, so the dosage and administration of 50mg has been approved in Japan.
Differences and comparisons with Olumiant
Although it is not possible to directly compare it with the similar drug Olumiant, I tried to arrange the results of the clinical trials that became the basis.
Although the average SALT score at enrollment was slightly higher with ALLEGRO-IIb/III, the efficacy was similar or slightly higher with LITFULO. Also, while the target age for Olumient is "18 years old and over", Lit Furo is "12 years old and over", so Lit Furo has a wider age range.
| BRAVE-AA2 | ALLEGRO-Ⅱb/Ⅲ | |||
| study arm * | PBO | Olumient 4mg | PBO | LITFULO 50mg group |
| Target age | 18+ | 12+ | ||
| Average age at enrollment (years) | 37.1 | 38.0 | 34.0 | 32.4 |
| Mean SALT score at enrollment | 85 | 84.8 | 93.0 | 90.3 |
| Proportion of SALT scores ≤20 at 24 weeks | 1.3% | 28.2% | 2% | twenty three% |
| Proportion of SALT scores ≤20 at 34 weeks | - | - | 9.23% ** | 33.87% |
| Proportion of SALT scores ≤20 at 36 weeks | 2.6% | 32.5% | - | - |
| Proportion of SALT scores ≤20 at Week 40 | - | - | 15.38% ** | 39.34% |
*PBO is the placebo group. There are other test groups, but the main dose groups are extracted. The primary endpoint of the BRAVE-AA1/BRAVE-AA2 study is "proportion of SALT score ≤20 at week 36" and the primary endpoint of ALLEGRO-IIb/III study is "proportion of SALT score ≤20 at week 24" .
**In the PBO arm of the ALLEGRO-IIb/III study, LITFULO 50mg has been administered from week 24 (extension phase).

For reference, I compared the attached documents and basic information.

It seems that there are differences in the pediatric indications and contraindications, as well as the profile of side effects.
side effects
As a serious side effect,
- Infectious diseases: herpes zoster (0.9%), oral herpes (0.8%), herpes simplex (0.5%), COVID-19 (0.2%), sepsis (0.1%)
- Lymphopenia (1.6%), thrombocytopenia (0.3%), hemoglobin decrease (0.2%), neutropenia (0.2%)
- Venous thromboembolism (incidence unknown)
- Liver dysfunction: ALT (0.9%), AST (0.5%)
- Bleeding: epistaxis (0.5%), positive urine (0.1%), contusion (0.1%)
etc. are listed, so be careful.
Summary/Afterword
This kind of medicine is Litfulo
- JAK3/TEC family kinase inhibitors for alopecia areata
- First TEC family kinase inhibitor in Japan
- Use once-daily orally
There has been a need for new treatment options for alopecia areata, as there have been few established evidence-based treatments.
In 2022, Olumient , a similar oral JAK inhibitor , will appear, expanding treatment options.
I hope that in the future they will consider how to properly use them! A list of oral JAK inhibitors is summarized below, so please refer to it as well.
This time, along with alopecia areata, I explained the mechanism of action and evidence of LITFULO (ritrecitinib) ! thank you!